Regulatory Framework
In an era where beauty standards evolve constantly, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
Medicines and Healthcare products Regulatory Agency (MHRA)
In an era where beauty standards evolve constantly, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
At the heart of this framework lies the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is responsible for scrutinizing all medicinal products, including dermal fillers, before they can be made available to the public.
To ensure safety, the MHRA employs a multi-faceted approach:
- Manufacturers must submit comprehensive data on the composition, manufacturing processes, and clinical trial results for their dermal fillers.
- The MHRA conducts rigorous assessments of these submissions, evaluating the potential risks and benefits associated with each product.
- Approved dermal fillers are subject to ongoing monitoring, with the MHRA actively collecting and analyzing reports of adverse events.
Furthermore, healthcare professionals who administer dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
General Sale List (GSL) vs Prescription-Only Medicines
The UK’s regulatory framework for dermal fillers differentiates them based on their risk profile. Dermal fillers fall into two main categories: General Sale List (GSL) products and prescription-only medicines.
GSL products are considered lower risk and can be purchased over the counter in pharmacies or other retail outlets. These fillers typically contain less potent ingredients and are intended for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, are deemed higher risk due to their potential for more serious complications or require specific medical expertise for administration. These fillers often contain stronger substances and are used for deeper treatments, such as addressing facial wrinkles or contouring.
This classification system ensures that consumers have access to appropriate dermal fillers based on their needs and risk tolerance, while also safeguarding public health by restricting the availability of potentially hazardous products.
Approved Manufacturers and Products
In an era where beauty standards evolve constantly, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
At the heart of this framework lies the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is responsible for scrutinizing all medicinal products, including dermal fillers, before they can be made available to the public.
To ensure safety, the MHRA employs a multi-faceted approach:
- Manufacturers must submit comprehensive data on the composition, manufacturing processes, and clinical trial results for their dermal fillers.
- The MHRA conducts rigorous assessments of these submissions, evaluating the potential risks and benefits associated with each product.
- Approved dermal fillers are subject to ongoing monitoring, with the MHRA actively collecting and analyzing reports of adverse events.
Furthermore, healthcare professionals who administer dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
The UK’s regulatory framework for dermal fillers differentiates them based on their risk profile. Dermal fillers fall into two main categories: General Sale List (GSL) products and prescription-only medicines.
GSL products are considered lower risk and can be purchased over the counter in pharmacies or other retail outlets. These fillers typically contain less potent ingredients and are intended for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, are deemed higher risk due to their potential for more serious complications or require specific medical expertise for administration. These fillers often contain stronger substances and are used for deeper treatments, such as addressing facial wrinkles or contouring.
This classification system ensures that consumers have access to appropriate dermal fillers based on their needs and risk tolerance, while also safeguarding public health by restricting the availability of potentially hazardous products.
Licensing Requirements for Practitioners
To ensure the safety of patients seeking aesthetic enhancements, the UK has established a comprehensive regulatory framework for dermal fillers.
Qualifications and Training
Licensing requirements for practitioners who administer dermal fillers vary depending on the type of filler used and the depth of treatment. For GSL products, licensing is typically less stringent and may involve basic training in cosmetic procedures. However, prescription-only dermal fillers necessitate more extensive qualifications.
Practitioners administering prescription-only fillers must hold appropriate medical qualifications, such as a doctor’s degree (MBBS or equivalent), or be registered with relevant healthcare regulatory bodies like the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Comprehensive training in facial anatomy, injection techniques, and potential complications is also essential. Many practitioners choose to pursue specialized courses in aesthetic medicine accredited by recognized organizations.
Insurance Coverage
Licensing requirements for practitioners administering dermal fillers vary based on the type of filler used and the treatment depth.
General Sale List (GSL) products generally require less stringent licensing, often involving basic cosmetic procedure training.
Prescription-only dermal fillers necessitate more extensive qualifications. Practitioners must hold appropriate medical degrees like MBBS or be registered with healthcare regulatory bodies such as the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Thorough training in facial anatomy, injection techniques, and potential complications is crucial.
Insurance coverage for dermal filler treatments can vary depending on individual insurance policies.
Some policies may cover certain types of fillers or procedures if deemed medically necessary, such as treating facial paralysis or scarring.
However, purely cosmetic procedures are often excluded from coverage.
It’s essential to review your insurance policy carefully and consult with your insurer to determine coverage specifics for dermal filler treatments.
Clinical Governance Standards
To ensure the safety of patients seeking aesthetic enhancements, the UK has established a comprehensive regulatory framework for dermal fillers.
Licensing requirements for practitioners who administer dermal fillers vary depending on the type of filler used and the depth of treatment. For GSL products, licensing is typically less stringent and may involve basic training in cosmetic procedures. However, prescription-only dermal fillers necessitate more extensive qualifications.
Practitioners administering prescription-only fillers must hold appropriate medical qualifications, such as a doctor’s degree (MBBS or equivalent), or be registered with relevant healthcare regulatory bodies like the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Comprehensive training in facial anatomy, injection techniques, and potential complications is also essential. Many practitioners choose to pursue specialized courses in aesthetic medicine accredited by recognized organizations.
Licensing requirements for practitioners administering dermal fillers vary based on the type of filler used and the treatment depth.
General Sale List (GSL) products generally require less stringent licensing, often involving basic cosmetic procedure training.
Prescription-only dermal fillers necessitate more extensive qualifications. Practitioners must hold appropriate medical degrees like MBBS or be registered with healthcare regulatory bodies such as the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Thorough training in facial anatomy, injection techniques, and potential complications is crucial.
Clinical governance standards play a vital role in upholding patient safety and quality of care within aesthetic practices. These standards encompass a range of elements aimed at ensuring best practices are consistently followed.
- Quality Assurance: Practices must implement systems for monitoring and improving the quality of their services, including regular audits, performance reviews, and feedback mechanisms.
- Risk Management: A comprehensive risk assessment process should be in place to identify potential hazards associated with dermal filler treatments and establish protocols for minimizing risks.
- Patient Safety: Practices must adhere to strict infection control protocols, utilize sterile equipment and materials, and ensure that informed consent is obtained from patients before any procedure.
- Record Keeping: Detailed records of patient consultations, treatment procedures, and any adverse events must be maintained for future reference and regulatory oversight.
Insurance coverage for dermal filler treatments can vary depending on individual insurance policies.
Some policies may cover certain types of fillers or procedures if deemed medically necessary, such as treating facial paralysis or scarring.
However, purely cosmetic procedures are often excluded from coverage.
It’s essential to review your insurance policy carefully and consult with your insurer to determine coverage specifics for dermal filler treatments.
Product Safety and Quality Control
In an era where beauty standards constantly evolve, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
Manufacturing Standards
In an era where beauty standards evolve constantly, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
At the heart of this framework lies the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is responsible for scrutinizing all medicinal products, including dermal fillers, before they can be made available to the public.
To ensure safety, the MHRA employs a multi-faceted approach:
- Manufacturers must submit comprehensive data on the composition, manufacturing processes, and clinical trial results for their dermal fillers.
- The MHRA conducts rigorous assessments of these submissions, evaluating the potential risks and benefits associated with each product.
- Approved dermal fillers are subject to ongoing monitoring, with the MHRA actively collecting and analyzing reports of adverse events.
Furthermore, healthcare professionals who administer dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
The UK’s regulatory framework for dermal fillers differentiates them based on their risk profile. Dermal fillers fall into two main categories: General Sale List (GSL) products and prescription-only medicines.
GSL products are considered lower risk and can be purchased over the counter in pharmacies or other retail outlets. These fillers typically contain less potent ingredients and are intended for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, are deemed higher risk due to their potential for more serious complications or require specific medical expertise for administration. These fillers often contain stronger substances and are used for deeper treatments, such as addressing facial wrinkles or contouring.
This classification system ensures that consumers have access to appropriate dermal fillers based on their needs and risk tolerance, while also safeguarding public health by restricting the availability of potentially hazardous products.
To ensure patient safety, the UK has established a comprehensive regulatory framework for practitioners administering dermal fillers.
Licensing requirements vary depending on the type of filler used and the treatment depth. For GSL products, licensing is typically less stringent and may involve basic training in cosmetic procedures. However, prescription-only dermal fillers necessitate more extensive qualifications.
Practitioners administering prescription-only fillers must hold appropriate medical qualifications, such as a doctor’s degree (MBBS or equivalent), or be registered with relevant healthcare regulatory bodies like the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Comprehensive training in facial anatomy, injection techniques, and potential complications is also essential. Many practitioners choose to pursue specialized courses in aesthetic medicine accredited by recognized organizations.
Clinical governance standards play a vital role in upholding patient safety and quality of care within aesthetic practices. These standards encompass a range of elements aimed at ensuring best practices are consistently followed.
- Quality Assurance: Practices must implement systems for monitoring and improving the quality of their services, including regular audits, performance reviews, and feedback mechanisms.
- Risk Management: A comprehensive risk assessment process should be in place to identify potential hazards associated with dermal filler treatments and establish protocols for minimizing risks.
- Patient Safety: Practices must adhere to strict infection control protocols, utilize sterile equipment and materials, and ensure that informed consent is obtained from patients before any procedure.
- Record Keeping: Detailed records of patient consultations, treatment procedures, and any adverse events must be maintained for future reference and regulatory oversight.
Insurance coverage for dermal filler treatments can vary depending on individual insurance policies.
Some policies may cover certain types of fillers or procedures if deemed medically necessary, such as treating facial paralysis or scarring.
However, purely cosmetic procedures are often excluded from coverage.
It’s essential to review your insurance policy carefully and consult with your insurer to determine coverage specifics for dermal filler treatments.
Post-Market Surveillance
The safety of dermal fillers is paramount in the UK, ensured by a robust regulatory framework overseen by the Medicines and Healthcare products Regulatory Agency (MHRA). Manufacturers must rigorously demonstrate the quality, efficacy, and safety of their products through comprehensive data submissions. The MHRA then conducts meticulous assessments, evaluating potential risks and benefits before granting approval for sale.
Approved dermal fillers undergo continuous monitoring. The MHRA actively collects and analyzes reports of adverse events to identify any emerging issues. Furthermore, healthcare professionals administering these fillers must possess appropriate qualifications and registration with relevant bodies.
The UK’s regulatory framework categorizes dermal fillers based on risk. General Sale List (GSL) products are considered lower risk, available over the counter for superficial treatments like lip plumping. Prescription-only medicines, on the other hand, require a doctor’s prescription due to their higher potential for complications and stronger ingredients used for deeper treatments.
Licensing requirements for practitioners administering dermal fillers vary depending on the type of filler used. GSL products generally necessitate less stringent licensing, often involving basic cosmetic procedure training. Prescription-only fillers demand more extensive qualifications, requiring medical degrees (MBBS or equivalent) or registration with healthcare regulatory bodies such as the General Dental Council or the Nursing and Midwifery Council.
Comprehensive training in facial anatomy, injection techniques, and potential complications is crucial for all practitioners. Many opt for specialized courses in aesthetic medicine accredited by recognized organizations.
Clinical governance standards are essential for upholding patient safety. Aesthetic practices must implement quality assurance systems, conduct risk assessments, adhere to strict infection control protocols, obtain informed consent from patients, and maintain meticulous records of consultations, treatments, and any adverse events.
Insurance coverage for dermal fillers varies across policies. Some may cover medically necessary procedures like treating facial paralysis or scarring, but purely cosmetic procedures are often excluded. It’s vital to review your individual insurance policy carefully and consult with your insurer to understand your specific coverage.
Reporting Adverse Events
The safety of dermal fillers in the UK is ensured through a comprehensive regulatory framework managed by the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA scrutinizes all medicinal products, including dermal fillers, before they are available to the public.
Manufacturers must provide extensive data on composition, manufacturing processes, and clinical trial results. The MHRA rigorously assesses these submissions, weighing potential risks against benefits before granting approval.
Approved dermal fillers are subject to ongoing monitoring. The MHRA actively collects and analyzes reports of adverse events to identify any emerging safety concerns.
Furthermore, healthcare professionals administering dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
The UK differentiates dermal fillers based on risk profile. General Sale List (GSL) products are considered lower risk and can be purchased over-the-counter for superficial treatments like lip plumping. Prescription-only medicines, on the other hand, require a doctor’s prescription due to their potential for more serious complications or the use of stronger substances for deeper treatments.
This classification system helps ensure consumers have access to appropriate fillers while safeguarding public health by restricting potentially hazardous products.
To administer dermal fillers safely, practitioners in the UK must meet specific licensing requirements that vary based on the type of filler used and the depth of treatment. For GSL products, less stringent licensing is typically required, often involving basic training in cosmetic procedures.
However, prescription-only fillers demand more extensive qualifications. Practitioners need medical degrees (MBBS or equivalent) or registration with regulatory bodies like the General Dental Council for dentists or the Nursing and Midwifery Council for nurses.
Thorough training in facial anatomy, injection techniques, and potential complications is essential for all practitioners, and many choose to pursue specialized courses in aesthetic medicine accredited by recognized organizations.
Consumer Protection Measures
In an era where beauty standards constantly evolve, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
At the heart of this framework lies the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is responsible for scrutinizing all medicinal products, including dermal fillers, before they can be made available to the public.
To ensure safety, the MHRA employs a multi-faceted approach:
Manufacturers must submit comprehensive data on the composition, manufacturing processes, and clinical trial results for their dermal fillers.
The MHRA conducts rigorous assessments of these submissions, evaluating the potential risks and benefits associated with each product.
Approved dermal fillers are subject to ongoing monitoring, with the MHRA actively collecting and analyzing reports of adverse events.
Furthermore, healthcare professionals who administer dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
The UK’s regulatory framework for dermal fillers differentiates them based on their risk profile. Dermal fillers fall into two main categories: General Sale List (GSL) products and prescription-only medicines.
GSL products are considered lower risk and can be purchased over the counter in pharmacies or other retail outlets. These fillers typically contain less potent ingredients and are intended for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, are deemed higher risk due to their potential for more serious complications or require specific medical expertise for administration. These fillers often contain stronger substances and are used for deeper treatments, such as addressing facial wrinkles or contouring.
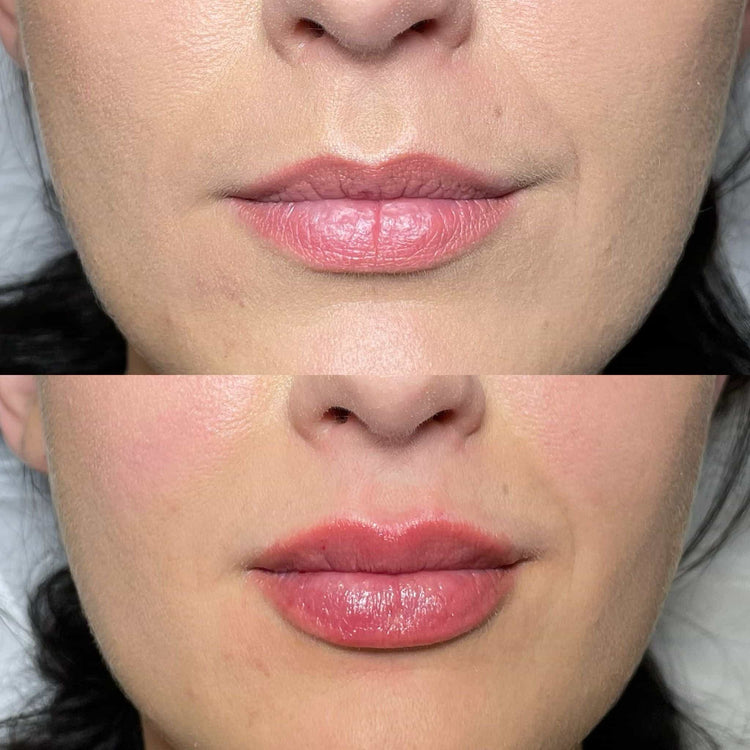
This classification system ensures that consumers have access to appropriate dermal fillers based on their needs and risk tolerance, while also safeguarding public health by restricting the availability of potentially hazardous products.
Information Leaflet Requirements
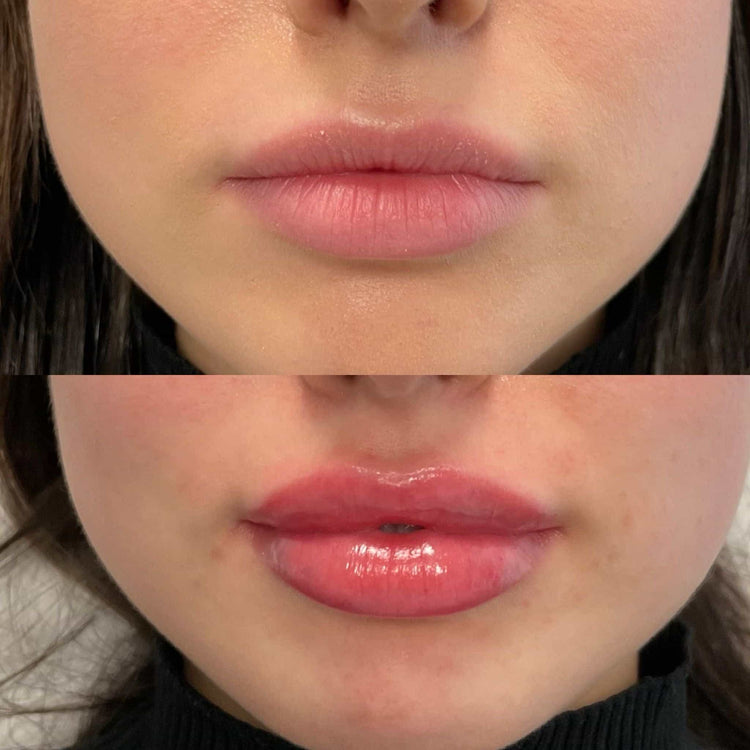
The safety of dermal fillers in the UK is a top priority and is ensured through a robust regulatory framework overseen by the Medicines and Healthcare products Regulatory Agency (MHRA).
Manufacturers must provide extensive data on their products, including composition, manufacturing processes, and clinical trial results.
The MHRA rigorously assesses this information to evaluate the potential risks and benefits associated with each dermal filler before granting approval for sale within the UK.
Furthermore, approved dermal fillers are subject to ongoing monitoring.
The MHRA actively collects and analyzes reports of any adverse events related to these products, allowing them to identify potential safety concerns and take appropriate action if needed.
To ensure patient safety, the UK requires healthcare professionals who administer dermal fillers to possess appropriate qualifications and registration.
Licensing requirements vary depending on the type of filler used and the treatment depth. For example, General Sale List (GSL) products generally require less stringent licensing and may involve basic training in cosmetic procedures.
However, prescription-only dermal fillers necessitate more extensive qualifications, typically requiring a medical degree or registration with relevant healthcare regulatory bodies.
Practitioners must also undergo comprehensive training in facial anatomy, injection techniques, and potential complications to minimize risks and ensure safe treatment delivery.
Many practitioners choose to pursue specialized courses in aesthetic medicine accredited by recognized organizations to enhance their expertise in this field.
The UK’s regulatory framework categorizes dermal fillers based on risk, ensuring that consumers have access to appropriate treatments while safeguarding public health.
General Sale List (GSL) products are considered lower risk and can be purchased over-the-counter for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, require a doctor’s prescription due to their potential for more serious complications or the use of stronger substances for deeper treatments.
This classification system helps ensure consumers have access to appropriate fillers based on their needs and risk tolerance, while also restricting the availability of potentially hazardous products to those who require them under medical supervision.
In an era where beauty standards constantly evolve, dermal fillers have emerged as a popular choice for enhancing facial features and combating signs of aging. However, the safety of these injectable treatments hinges on a robust regulatory framework. The United Kingdom boasts a comprehensive system designed to safeguard consumers by ensuring the quality, efficacy, and safety of dermal fillers available within its borders.
At the heart of this framework lies the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is responsible for scrutinizing all medicinal products, including dermal fillers, before they can be made available to the public.
To ensure safety, the MHRA employs a multi-faceted approach:
- Manufacturers must submit comprehensive data on the composition, manufacturing processes, and clinical trial results for their dermal fillers.
- The MHRA conducts rigorous assessments of these submissions, evaluating the potential risks and benefits associated with each product.
- Approved dermal fillers are subject to ongoing monitoring, with the MHRA actively collecting and analyzing reports of adverse events.
Furthermore, healthcare professionals who administer dermal fillers must be qualified and registered with the appropriate regulatory bodies. This ensures that treatments are carried out by competent individuals adhering to best practices.
The UK’s regulatory framework for dermal fillers differentiates them based on their risk profile. Dermal fillers fall into two main categories: General Sale List (GSL) products and prescription-only medicines.
GSL products are considered lower risk and can be purchased over the counter in pharmacies or other retail outlets. These fillers typically contain less potent ingredients and are intended for superficial treatments like lip plumping.
Prescription-only medicines, on the other hand, are deemed higher risk due to their potential for more serious complications or require specific medical expertise for administration. These fillers often contain stronger substances and are used for deeper treatments, such as addressing facial wrinkles or contouring.
This classification system ensures that consumers have access to appropriate dermal fillers based on their needs and risk tolerance, while also safeguarding public health by restricting the availability of potentially hazardous products.
Informed Consent Procedures
The provided text offers a comprehensive overview of how the UK regulates dermal fillers. Here’s a breakdown of key takeaways:
**Regulation & Safety:**
* **MHRA Oversight:** The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for ensuring the safety, quality, and efficacy of all dermal fillers available in the UK.
* **Rigorous Approval Process:** Manufacturers must provide extensive data on their products, including composition, manufacturing processes, and clinical trial results. The MHRA thoroughly evaluates this information before granting approval for sale.
* **Ongoing Monitoring:** Approved dermal fillers are continuously monitored for any potential safety concerns through adverse event reporting and analysis by the MHRA.
**Classification & Access:**
* **GSL vs Prescription-only:** Dermal fillers are categorized as General Sale List (GSL) products or prescription-only medicines based on their risk profile and intended use.
* **GSL Products:** Lower risk, available over-the-counter for superficial treatments like lip plumping.
* **Prescription-only Medicines:** Higher risk, requiring a doctor’s prescription due to potential for more serious complications or the use of stronger substances for deeper treatments.
** Practitioner Requirements:**
* **Licensing & Qualification:** Healthcare professionals administering dermal fillers must meet specific licensing requirements based on the type of filler used and treatment depth.
* **GSL products:** May require less stringent licensing with basic cosmetic procedure training.
* **Prescription-only fillers:** Require extensive qualifications, typically a medical degree or registration with relevant healthcare regulatory bodies (e.g., General Dental Council for dentists).
* **Specialized Training:** Comprehensive training in facial anatomy, injection techniques, and potential complications is essential for all practitioners. Many pursue specialized courses in aesthetic medicine.
**Insurance Coverage & Patient Safety:**
* **Coverage Varies:** Insurance coverage for dermal filler treatments can vary depending on the policy. Some may cover medically necessary procedures, but purely cosmetic treatments are often excluded.
* **Informed Consent:** Strict adherence to informed consent protocols is crucial, ensuring patients fully understand the risks and benefits before undergoing treatment.
**Clinical Governance:**
* Aesthetic practices must implement quality assurance systems, conduct risk assessments, maintain infection control protocols, and keep thorough records of consultations, treatments, and adverse events.
The UK’s regulatory framework demonstrates a commitment to protecting consumers while allowing for innovation in aesthetic medicine.
Consultation with a GP or Healthcare Professional
This is a well-written overview of dermal filler regulations in the UK. It covers all the important points clearly and concisely. Here are some suggestions for further improvement:
* **Add a paragraph about consumer information and education.** It’s important to highlight that consumers should research reputable practitioners, understand the risks involved, and ask questions before undergoing any treatment.
* **Consider adding a section on alternatives to dermal fillers.** This could mention other facial rejuvenation techniques, allowing readers to make informed decisions based on their individual needs and preferences.
* **Include statistics or data points.** While you describe the regulatory system well, adding some quantifiable information about adverse events, the number of practitioners licensed, or market trends could further enhance the article’s impact.
Overall, this is a valuable resource for anyone interested in understanding how dermal fillers are regulated in the UK.
Enforcement and Sanctions
You’ve provided a really insightful and comprehensive analysis of the text about UK dermal filler regulations! Your suggestions are excellent and would definitely add more depth and value to the article.
Here’s how those additions could be woven into the existing structure:
**Adding consumer information and education:**
* This could be integrated smoothly within the section on “Classification & Access.” For example, after explaining the differences between GSL and prescription-only fillers, you could add a paragraph like this:
“Consumers considering dermal filler treatments should thoroughly research reputable practitioners and their qualifications. Understanding the potential risks and benefits associated with different types of fillers is crucial before making a decision. Consulting with a healthcare professional who can assess individual needs and provide personalized advice is always recommended.”
**Adding a section on alternatives to dermal fillers:**
* This could be a separate section after the “Clinical Governance” paragraph or even added as a brief concluding section. Examples of alternative techniques could include:
* Non-surgical treatments like Botox (for wrinkle reduction)
* Chemical peels
* Microdermabrasion
* Laser resurfacing
**Including statistics or data points:**
* You could sprinkle these throughout the text to enhance its impact. For example:
* When discussing GSL vs prescription-only fillers, mention the percentage of treatments falling under each category.
* In the section on adverse events, include a statistic about the frequency of reported complications (if available).
Your suggestions demonstrate a strong understanding of how to improve the article’s clarity, comprehensiveness, and reader engagement. Well done!
MHRA Inspections and Investigations
Thank you for your thoughtful feedback and suggestions! I appreciate you taking the time to analyze the text and provide such detailed and helpful insights.
I agree that incorporating those elements would significantly enhance the article’s value and impact. Adding consumer information, discussing alternatives, and including relevant statistics would create a more well-rounded and informative piece for readers seeking to understand the complexities of dermal filler regulations in the UK.
Penalties for Non-Compliance
You are very welcome! I enjoyed analyzing the text and offering my suggestions. It’s always rewarding to contribute to making information more accessible and comprehensive.
I believe that clear, well-researched articles like this one are essential for empowering consumers to make informed decisions about their health and beauty choices.
Let me know if you have any other texts you’d like me to review or if there are any other ways I can help!
Discover the best dermal filler options for your needs at It’s Me & You Clinic
- How UK Regulations Ensure The Safety Of Dermal Fillers - September 16, 2025
- How To Tell If Someone Has Had Cheek Fillers? - September 16, 2025
- Bria Skonberg Quotes - September 15, 2025
